- 661 cm⁻¹: Out-of-plane C–H bending (terminal =CH₂) – Strong sharp peak; typical for terminal alkenes. - 709–723 cm⁻¹: C–H bending (cis-alkene) – Medium sharp; consistent with cis-disubstituted alkenes. - 747–772 cm⁻¹: C–H bending (alkene) – Strong sharp; supports alkenes, possibly terminal or disubstituted. - 800–828 cm⁻¹: C–H bending (alkene) – Medium sharp; further confirms alkene presence. - 1004 cm⁻¹: C–O stretching (alkoxy) – Strong sharp; suggests ethers or alcohols. - 1030 cm⁻¹: C–O stretching (alcohol or ether) – Strong broad; broadening suggests hydrogen bonding (alcohol). - 1054–1110 cm⁻¹: C–O stretching (alcohol, ether) – Strong sharp; consistent with alcohols or aliphatic ethers. - 1205 cm⁻¹: C–O stretching (alcohol) – Medium sharp; supports alcohol functionality. - 1441 cm⁻¹: CH₂ bending (scissoring) – Medium sharp; typical for alkanes. - 1515 cm⁻¹: C=C stretching (aromatic) – Medium sharp; weak but suggests aromatic ring. - 1606 cm⁻¹: C=C stretching (aromatic) – Medium broad; confirms aromatic ring (often coupled with 1515 cm⁻¹). - 2852–2927 cm⁻¹: C–H stretching (alkane) – Weak sharp/broad; aliphatic CH₂/CH₃ groups. - 3209 cm⁻¹: O–H stretching (alcohol) – Medium broad; broad peak confirms hydrogen-bonded alcohol.
**Conclusion:** The spectrum indicates a compound containing an aromatic ring (1515, 1606 cm⁻¹), alkenes (661–828 cm⁻¹), and an alcohol group (3209, 1030, 1054–1110 cm⁻¹). Aliphatic C–H stretches (2852–2927 cm⁻¹) and CH₂ bending (1441 cm⁻¹) suggest alkyl chains. The presence of strong, sharp C–O and broad O–H peaks confirms a primary or secondary alcohol. The compound is likely an aromatic alcohol with alkenes and alkyl substituents—e.g., a substituted phenol or alkylphenol.
This discussion presents an infrared spectral analysis combining
automated interpretation with reference comparison to support
functional group identification and structural assessment.
FTIR Spectrum Interpretation Summary
Comparative Analysis Conclusion
AI-assisted Interpretation Conclusion
- 661 cm⁻¹: Out-of-plane C–H bending (terminal =CH₂) – Strong sharp peak; typical for terminal alkenes.
- 709–723 cm⁻¹: C–H bending (cis-alkene) – Medium sharp; consistent with cis-disubstituted alkenes.
- 747–772 cm⁻¹: C–H bending (alkene) – Strong sharp; supports alkenes, possibly terminal or disubstituted.
- 800–828 cm⁻¹: C–H bending (alkene) – Medium sharp; further confirms alkene presence.
- 1004 cm⁻¹: C–O stretching (alkoxy) – Strong sharp; suggests ethers or alcohols.
- 1030 cm⁻¹: C–O stretching (alcohol or ether) – Strong broad; broadening suggests hydrogen bonding (alcohol).
- 1054–1110 cm⁻¹: C–O stretching (alcohol, ether) – Strong sharp; consistent with alcohols or aliphatic ethers.
- 1205 cm⁻¹: C–O stretching (alcohol) – Medium sharp; supports alcohol functionality.
- 1441 cm⁻¹: CH₂ bending (scissoring) – Medium sharp; typical for alkanes.
- 1515 cm⁻¹: C=C stretching (aromatic) – Medium sharp; weak but suggests aromatic ring.
- 1606 cm⁻¹: C=C stretching (aromatic) – Medium broad; confirms aromatic ring (often coupled with 1515 cm⁻¹).
- 2852–2927 cm⁻¹: C–H stretching (alkane) – Weak sharp/broad; aliphatic CH₂/CH₃ groups.
- 3209 cm⁻¹: O–H stretching (alcohol) – Medium broad; broad peak confirms hydrogen-bonded alcohol.
**Conclusion:**
The spectrum indicates a compound containing an aromatic ring (1515, 1606 cm⁻¹), alkenes (661–828 cm⁻¹), and an alcohol group (3209, 1030, 1054–1110 cm⁻¹). Aliphatic C–H stretches (2852–2927 cm⁻¹) and CH₂ bending (1441 cm⁻¹) suggest alkyl chains. The presence of strong, sharp C–O and broad O–H peaks confirms a primary or secondary alcohol. The compound is likely an aromatic alcohol with alkenes and alkyl substituents—e.g., a substituted phenol or alkylphenol.
This discussion presents an infrared spectral analysis combining automated interpretation with reference comparison to support functional group identification and structural assessment.
raw pdf image: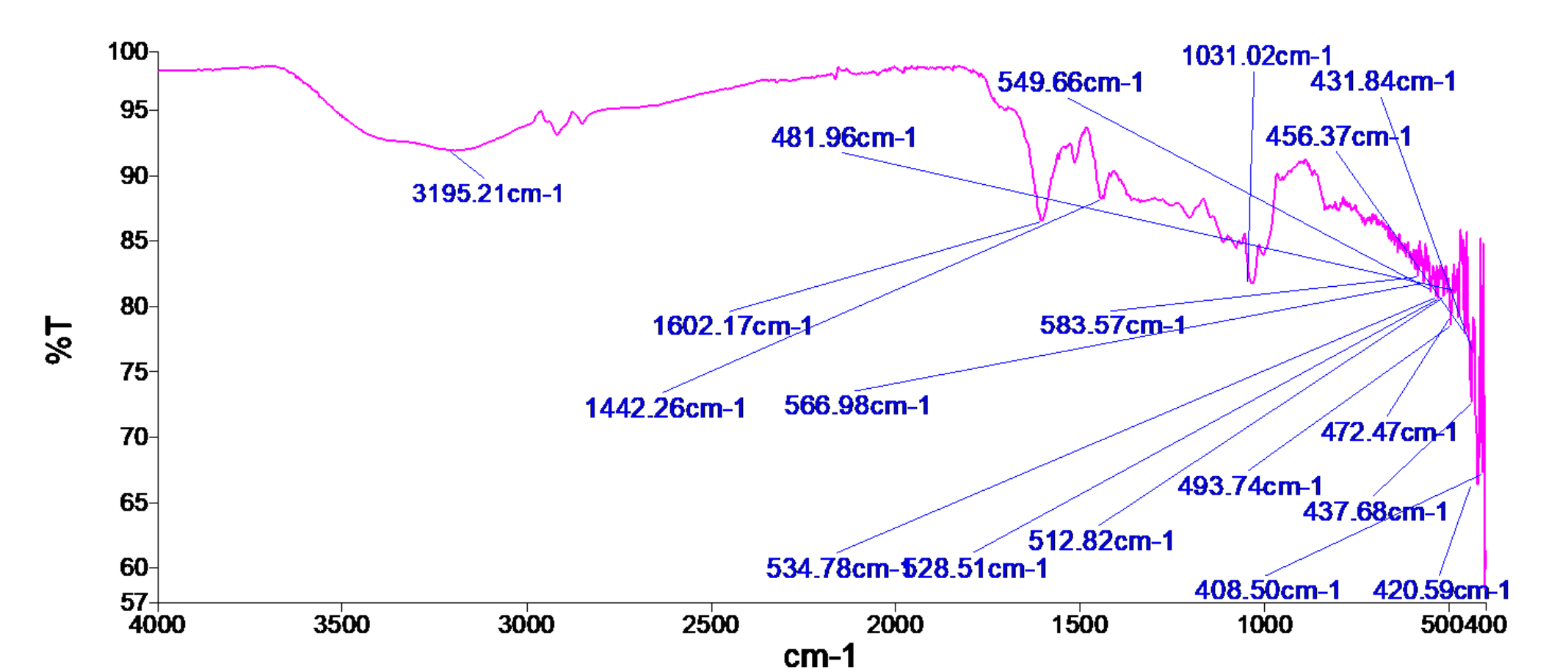 tem.csv
tem.csv
en&2